When a pharmacist hands you a pill bottle labeled NTI generics, they’re not just filling a prescription-they’re making a clinical decision with real consequences. These aren’t your average generic drugs. For medications with a narrow therapeutic index, even tiny changes in how the body absorbs the drug can mean the difference between healing and harm. And pharmacists are sounding the alarm.
What Exactly Are NTI Generics?
NTI stands for Narrow Therapeutic Index. These are drugs where the gap between an effective dose and a toxic one is razor-thin. A 10% shift in blood concentration might mean the drug works perfectly-or it could trigger seizures, internal bleeding, or organ failure. Common examples include warfarin, levothyroxine, phenytoin, carbamazepine, and cyclosporine. The FDA doesn’t publish an official list, but it does flag them in the Orange Book with special codes. Pharmacists know these drugs by heart because they’ve seen what happens when things go wrong.
Unlike regular generics, which must be within 80-125% of the brand drug’s absorption rate, NTI generics are held to a tighter standard: 90-111%. That might sound strict, but it’s still wide enough to cause problems. In 2024, the University of Minnesota found 42 drugs with recommended narrower bioequivalence ranges-and 15 of them had documented cases of harm when substitutions weren’t controlled.
Why Pharmacists Are Worried
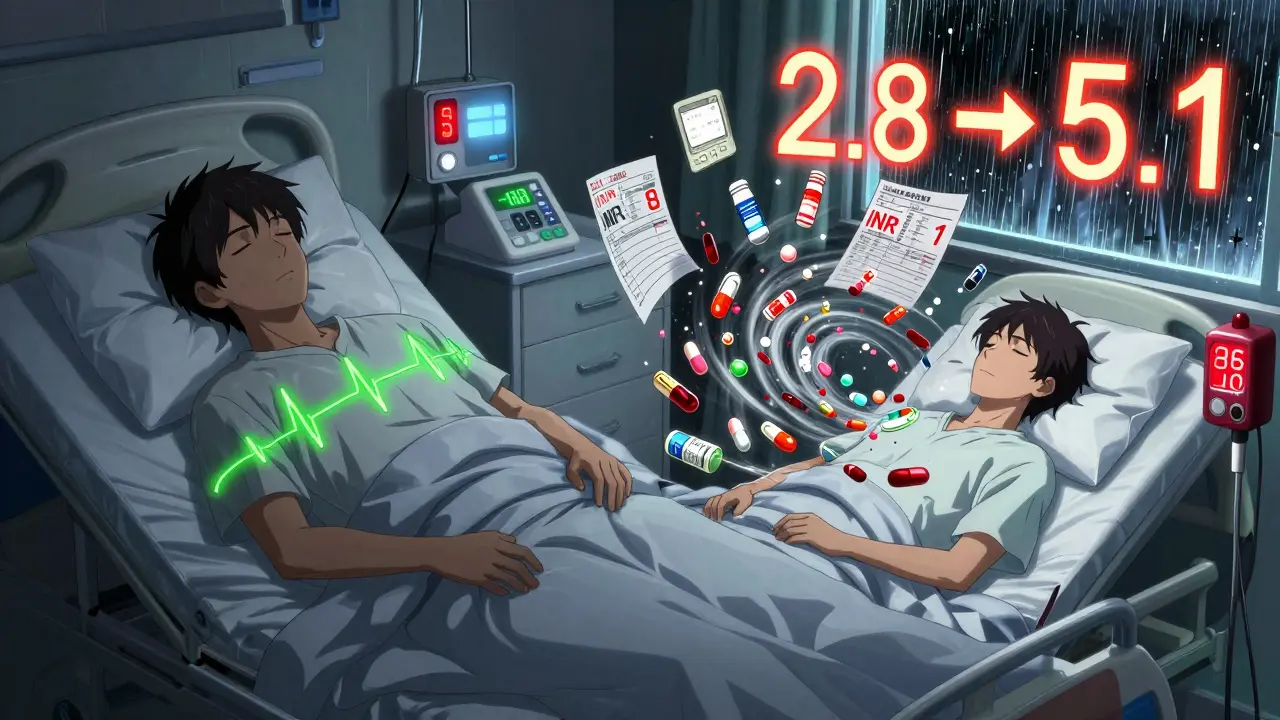
It’s not about distrust in generics. It’s about consistency. When a patient switches from one generic manufacturer to another-even if both are FDA-approved-their blood levels can fluctuate. One pharmacist in Melbourne told me about a patient on warfarin who went from an INR of 2.8 to 5.1 after switching generic brands. She ended up in the ER with a bleeding ulcer. That’s not rare. Between 2020 and 2024, the FDA’s adverse event database logged 1,247 incidents tied to NTI generic switches. For non-NTI generics? Just 382.
Surveys show the concern is widespread. In 2024, 68% of pharmacists surveyed by ASHP said they worry about NTI substitution. In community pharmacies, 73% reported doctors asking them to avoid switching NTI generics altogether. Warfarin leads the list, followed by levothyroxine and carbamazepine. These aren’t hypothetical fears. They’re based on real patient outcomes.
The Cost-Saving Trap
Generics save money-no doubt about it. NTI generics cost 80-85% less than brand versions. That’s why insurers push them. But here’s the catch: cost savings can come at the cost of stability. One independent pharmacy owner saw a 35% drop in patients abandoning their prescriptions when generics became available. That’s great-until the same patient starts having erratic INR levels because they switched from one generic to another every month.
Drug shortages make it worse. As of Q1 2025, NTI drugs made up 17% of all active shortages, even though they account for only 6% of generic prescriptions. Why? Because when a single manufacturer has a production issue, there’s often no backup. And switching between different generic brands mid-treatment? That’s when things get dangerous.
State Laws Are a Patchwork
There’s no national rule on NTI substitution. As of January 2025, only 28 states have laws restricting automatic substitution for NTI drugs. In 22 states, pharmacists must notify the prescriber before switching. Six states ban it outright. The rest? No restrictions. That means a patient in one state might get a stable, unswitched prescription-and in the next state, their medication changes without anyone telling them.
Pharmacists are caught in the middle. They know what’s safest, but they’re legally required to dispense what’s cheapest unless the prescriber blocks it. And many prescribers don’t even know the difference between a regular generic and an NTI generic. That’s why 61% of pharmacists say they want state laws to require prescriber approval before any NTI substitution.
What Pharmacists Are Doing About It
Forward-thinking hospitals and clinics are building protocols. UCSF’s 2024 guidelines recommend a 48-hour learning curve for staff: train on therapeutic drug monitoring, document every switch, and never let patients bounce between generic brands. The ASHP’s 2025 toolkit says: if you can, stick to one manufacturer. Sixty-three percent of hospital systems now do this. It’s not ideal, but it’s safer.
Pharmacists are also stepping up their education. In 2024, 81% of pharmacy residency programs added NTI-specific training. That’s up from 42% just five years ago. They’re learning how to interpret drug levels, recognize early signs of instability, and communicate with patients in plain language. “I tell them,” one pharmacist said, “that this isn’t like switching painkillers. This is like tuning a piano. A little off, and the whole song breaks.”
The Bigger Picture: Supply Chains and Regulation
Most generics-especially NTI drugs-are made overseas. The University of Minnesota found 80% of generics are finished in foreign countries, and NTI drugs are even more reliant on overseas production. That means supply chain shocks from weather, politics, or factory inspections can trigger shortages. The FTC is now investigating how group purchasing organizations contribute to the instability, especially for NTI drugs.
The FDA is listening. In April 2025, they announced a new bioequivalence framework for 12 high-priority NTI drugs, with stricter standards coming by 2026. But experts are skeptical. Dr. Lucinda Maine of the American Association of Colleges of Pharmacy said, “We’ve been here before. The science says we need tighter controls, but the system moves slowly.”
Meanwhile, Medicare’s new drug price negotiation program includes three NTI drugs among its first 10 targets. Pharmacists fear the 21-day reimbursement delay could force community pharmacies to stop stocking these drugs altogether-just when patients need them most.
What Patients Should Know
If you’re on warfarin, levothyroxine, or another NTI drug, here’s what you need to do:
- Ask your pharmacist: “Is this the same brand as last time?”
- Check your blood levels regularly-don’t skip tests.
- If you feel different after a refill-fatigue, dizziness, irregular heartbeat-call your doctor immediately.
- Ask your prescriber to write “Dispense as Written” or “Do Not Substitute” on the prescription.
It’s not about being difficult. It’s about staying safe.
What’s Next?
The future of NTI generics isn’t clear. But one thing is: pharmacists aren’t going to stop speaking up. By 2027, 74% of healthcare systems plan to put pharmacists in charge of NTI drug stewardship programs. That means pharmacists will have a formal role in approving substitutions, not just dispensing them.
For now, the system is a balancing act. Generics keep drugs affordable. But for NTI drugs, affordability can’t come at the cost of safety. Until the bioequivalence standards catch up with the science, and until supply chains become more reliable, the safest choice remains: consistency. One brand. One dose. One pharmacist who knows the stakes.
Are all generic drugs the same as brand-name drugs?
No-not all generics are created equal. For most medications, generics work just as well. But for drugs with a narrow therapeutic index (NTI), like warfarin or levothyroxine, even small differences in absorption can lead to serious side effects or treatment failure. These drugs require stricter controls and often need to come from the same manufacturer to maintain stability.
Can I switch between different NTI generic brands safely?
It’s not recommended. Even though each generic meets FDA standards, switching between manufacturers can cause your blood levels to fluctuate. For drugs like warfarin, that can mean dangerous bleeding or clotting. If you’ve been stable on one brand, ask your pharmacist and doctor to keep you on it. Don’t let automatic substitution happen without your knowledge.
Why do some states block NTI generic substitution?
Because patient safety outweighs cost savings in these cases. States with restrictions have seen fewer adverse events linked to NTI drug switches. In places like California and New York, pharmacists must get prescriber approval before substituting NTI drugs. These laws exist because real people have been harmed by well-intentioned cost-cutting.
How do I know if my medication is an NTI drug?
Ask your pharmacist or check the drug’s label. Common NTI drugs include warfarin, levothyroxine, phenytoin, carbamazepine, cyclosporine, and digoxin. If your doctor says you need regular blood tests to monitor your levels, that’s a strong sign you’re on an NTI drug. Don’t assume it’s just a regular generic.
What should I do if I notice side effects after switching generics?
Contact your doctor right away. Don’t wait. Symptoms like unusual bruising, fatigue, confusion, heart palpitations, or seizures could signal a dangerous change in drug levels. Bring your pill bottle with you-your doctor will need to know which generic brand you’re taking. You may need a blood test and possibly a switch back to your original brand.
Is there a list of NTI drugs I can check?
The FDA doesn’t publish a public list, but the Orange Book identifies drugs with therapeutic equivalence codes that signal potential substitution risks. Pharmacists use this database daily. You can ask your pharmacist to look up your drug’s code. Resources like the AAM’s NTI Drug Information Portal also offer guidance for patients and providers.
Why are NTI drugs more prone to shortages?
Because they’re made by fewer manufacturers, often overseas, and require more complex production. If one factory has a problem, there’s rarely a quick replacement. The FDA reported 47 NTI drug shortages in 2024-nearly one in five of all shortages. This isn’t random; it’s systemic. And when a shortage hits, patients get switched between brands, increasing risk.
Final Thoughts
NTI generics aren’t bad. They’re necessary. But they’re not simple. Behind every bottle is a patient who depends on consistency. Behind every pharmacist’s hesitation is years of experience watching lives hang in the balance. The system needs to evolve-tighter standards, better supply chains, clearer rules. Until then, the best defense is awareness: know your drug, know your brand, and never stop asking questions.




Pharmacists are the unsung heroes of the healthcare system, and this post nails it. I’ve seen patients on levothyroxine go from feeling fine to exhausted and depressed after a switch - no one told them it happened. It’s not about fear of generics; it’s about respecting the science. Consistency saves lives.