Alzheimer’s disease affects more than 55 million people worldwide, and the number is climbing as populations age. While there’s no cure yet, one drug has become a cornerstone of treatment: Donepezil is a reversible acetylcholinesterase inhibitor approved for mild to moderate Alzheimer’s disease. People often wonder how this pill fits into the broader picture of brain health and whether it can truly slow cognitive decline. This guide breaks down the science, the benefits, the risks, and the everyday steps you can take to support a sharper mind.
Key Takeaways
- Donepezil works by boosting acetylcholine, a neurotransmitter crucial for learning and memory.
- Clinical studies show modest improvements in cognition and daily functioning for many patients.
- Side effects are generally mild but can include nausea, insomnia, and muscle cramps.
- Regular monitoring by a health professional and lifestyle support are essential for best outcomes.
- Alternative medications such as Rivastigmine and Memantine target the same pathways with different profiles.
How Donepezil Works in the Brain
The brain relies on a balanced chemistry of neurotransmitters. Acetylcholinesterase inhibitor a class of drugs that blocks the enzyme breaking down acetylcholine prevents the rapid degradation of this key messenger. Acetylcholine supports attention, learning, and memory formation. In Alzheimer’s, the cholinergic system - the network of neurons that uses acetylcholine - deteriorates, leading to the hallmark memory loss.
By inhibiting the enzyme, donepezil raises acetylcholine levels, allowing remaining cholinergic neurons to function more effectively. The result is a modest boost in synaptic activity that can translate into better performance on cognitive tests and everyday tasks.
Clinical Benefits for Brain Health
Large‑scale trials led by the FDA the U.S. Food and Drug Administration, the agency that approves medications and independent research groups have documented donepezil’s impact. On average, patients experience a 2‑3‑point improvement on the Mini‑Mental State Examination (MMSE) after six months of treatment compared to placebo. While this may sound small, it often means the difference between needing constant supervision and maintaining some independence.
Beyond test scores, real‑world benefits include:
- Reduced agitation and improved mood.
- Better performance in activities of daily living such as dressing and meal preparation.
- Delaying institutionalization by several months to a year in many cases.
It’s important to set realistic expectations: donepezil does not halt disease progression, but it can smooth the decline curve.
Who Might Benefit?
Donepezil is approved for mild to moderate Alzheimer’s, but clinicians sometimes prescribe it off‑label for other forms of cognitive impairment, like Cognitive decline the gradual loss of mental abilities associated with aging or disease. Ideal candidates usually:
- Have a confirmed diagnosis of Alzheimer’s via clinical evaluation and imaging.
- Display measurable memory or executive function deficits.
- Do not have contraindications such as severe heart block or active peptic ulcer disease.
Discussion with a neurologist or geriatric psychiatrist helps tailor the decision to personal health status, caregiver support, and quality‑of‑life goals.
Dosage, Administration, and Monitoring
Standard dosing starts at 5 mg once daily, often taken at bedtime to reduce nausea. After four to six weeks, the dose may be increased to 10 mg, and for select patients, a 23 mg extended‑release tablet is an option after a minimum of three months on 10 mg.
Regular follow‑up visits (every 3‑6 months) are crucial to assess:
- Cognitive test scores (MMSE or MoCA).
- Side‑effect profile.
- Weight, heart rate, and liver function.
Any worsening of symptoms or intolerable side effects should prompt dosage adjustment or a switch to an alternative.
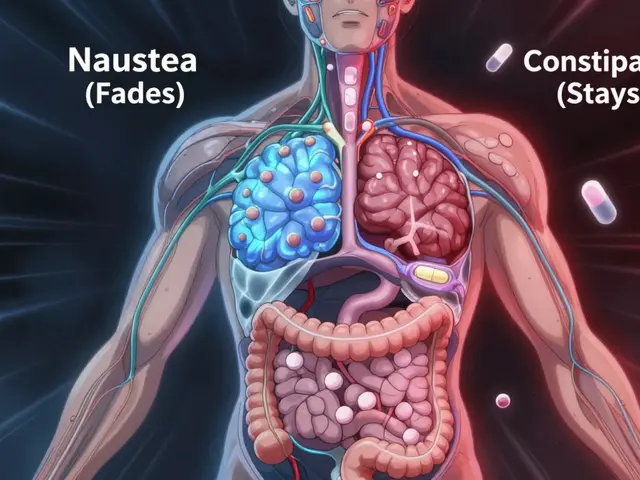
Common Side Effects and Safety Concerns
Most patients tolerate donepezil well. The most frequent adverse events are:
- Nausea and loss of appetite.
- Insomnia or vivid dreams.
- Muscle cramps, especially in the lower limbs.
- Occasional dizziness or fainting due to bradycardia.
Serious but rare complications include severe bradyarrhythmias and gastrointestinal bleeding. Patients on anticoagulants or non‑steroidal anti‑inflammatory drugs (NSAIDs) should be monitored closely.

Alternatives and Combination Therapies
When donepezil is ineffective or poorly tolerated, clinicians may consider other acetylcholinesterase inhibitors or drugs with different mechanisms:
| Drug | Class | Typical Dose | Key Differences |
|---|---|---|---|
| Donepezil | Acetylcholinesterase inhibitor | 5‑10 mg daily (23 mg extended‑release) | Once‑daily dosing, longest‑acting among its class |
| Rivastigmine a carbamate‑based acetylcholinesterase inhibitor | Acetylcholinesterase inhibitor | 1.5‑6 mg twice daily (patch: 4.6‑9.5 mg/24 h) | Available as oral and transdermal patch; higher GI side‑effects |
| Memantine an NMDA receptor antagonist | Glutamate regulator | 5‑20 mg daily | Targets excitotoxicity; often combined with donepezil for moderate‑to‑severe stages |
Combination therapy (e.g., donepezil + memantine) is supported by several studies showing additive benefits in later disease stages.
Lifestyle Strategies to Complement Medication
Medication works best when paired with brain‑friendly habits. Consider these evidence‑based tips:
- Regular aerobic exercise (150 minutes/week) boosts cerebral blood flow.
- Balanced diet rich in omega‑3 fatty acids, berries, leafy greens, and low in saturated fat.
- Cognitive training through puzzles, learning a new language, or musical instrument.
- Quality sleep - aim for 7‑8 hours to allow glymphatic clearance of toxic proteins.
- Social engagement reduces stress hormones that can accelerate neurodegeneration.
Caregivers play a pivotal role: monitoring adherence, noting changes, and encouraging these habits.
Frequently Asked Questions
Can Donepezil prevent Alzheimer’s?
No. Donepezil does not stop the disease from starting. It temporarily improves neurotransmitter function, which can delay symptom worsening for months to a few years.
How long should I stay on Donepezil?
Most clinicians continue treatment as long as cognitive benefits outweigh side effects. Regular reviews help decide when to taper or stop.
Is it safe to take Donepezil with other medicines?
Donepezil can interact with anticholinergic drugs (e.g., certain antihistamines) and medications that affect heart rhythm. Always inform your prescriber of every drug and supplement you use.
What should I do if I miss a dose?
Take the missed dose as soon as you remember, unless it’s near the time of the next scheduled dose. In that case, skip the missed one and resume the regular schedule. Never double‑dose.
Are there natural alternatives to Donepezil?
Some plant extracts (e.g., Huperzine A) have mild acetylcholinesterase‑inhibiting properties, but evidence is limited and quality control varies. Discuss any supplement with a healthcare professional.
Understanding the link between donepezil and brain health empowers patients and families to make informed choices. While the drug isn’t a miracle cure, it offers a valuable tool when used correctly and combined with healthy lifestyle habits.





Donepezil remains a cornerstone in the pharmacologic management of mild to moderate Alzheimer’s disease.
Its mechanism of action-reversible inhibition of acetylcholinesterase-directly augments synaptic acetylcholine, a neurotransmitter essential for memory encoding.
Clinical trials have consistently demonstrated modest yet statistically significant improvements on standardized cognitive scales such as the MMSE and ADAS‑Cog.
It is important to recognize that these gains are typically observed within the first six to twelve months of therapy.
Patients who adhere to the prescribed regimen frequently report enhanced ability to perform instrumental activities of daily living.
Moreover, caregivers often note a reduction in agitation and a more stable mood profile in the treated individual.
Nevertheless, donepezil does not arrest the underlying neurodegenerative process, and disease progression continues, albeit at a slower trajectory.
Appropriate patient selection-confirmed diagnosis, absence of contraindicating cardiac conduction abnormalities, and realistic expectations-is essential for optimal outcomes.
Dose titration usually begins with 5 mg nightly, advancing to 10 mg after four to six weeks as tolerated.
For certain patients who have demonstrated stability on 10 mg, the 23 mg extended‑release formulation may be considered after a minimum three‑month trial.
Routine monitoring should include periodic cognitive assessment, weight measurement, heart rate, and liver function tests.
Side effects are generally mild, with nausea, insomnia, and muscle cramps being the most frequently reported.
In the event of severe bradycardia or gastrointestinal bleeding, immediate medical review is warranted.
Complementary lifestyle interventions-regular aerobic exercise, a Mediterranean‑type diet, and sustained cognitive engagement-synergize with pharmacotherapy.
Caregiver education about medication adherence and symptom tracking further enhances the therapeutic benefit.
In summary, when prescribed judiciously and combined with holistic brain‑health strategies, donepezil can meaningfully improve quality of life for many patients and their families.